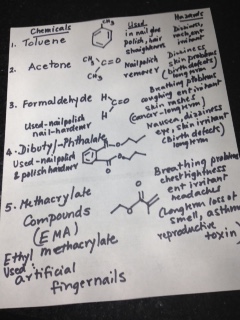
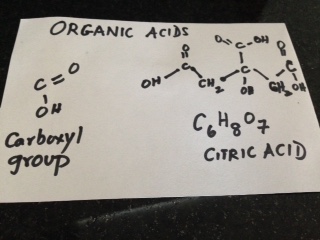
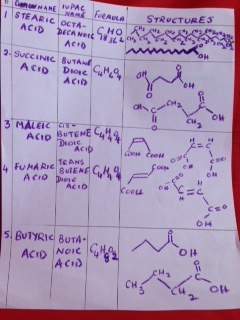
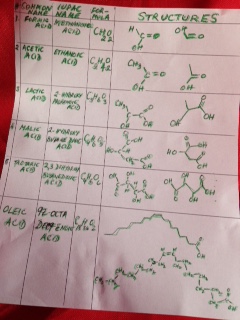
When we were growing up in India, power failures were common and we all had candles and matchboxes handy. The sides of those little boxes had a brownish- red tint and we struck our little match – heads on the sides to light them and light up candles. Later, we saved the match- boxes to play games. We made miniature furniture, or used them to save our trinkets and little stickers.
The match- heads had phosphorus. The chemical symbol is P and it is the 15th element in the Periodic Table. It is part of the Nitrogen Group, in between Silicon and Sulfur. Next to Calcium, Ca, Phosphorus is the most abundant element in the body. (Ref.1) It is essential in the building blocks of our bones and genes,viz.,the DNA and RNA. Actually most of us have more P than we need since it is found in a lot of foods that we eat.
Phosphorus was discovered in 1669 while working with urine! Hennig Brand, a German alchemist, heated the residue left behind after the evaporation of urine. Elemental phosphorus was discovered. In nature, one never finds elemental P but it is always found as rock phosphates. (Ref. 2) A hundred years later, it was extracted using bone , which has become the traditional way of chemically producing phosphorus. (Ref. 3)
Phosphorus, P, exists in 3 allotropic forms, red, white and black. Red and white are more common. (Nuggets of Information) Both red and white P are poly-atomic and are 4 P atoms that form a tetrahedron. (Fig.3) White phosphorus reacts vigorously with oxygen and the reaction results in a popping sound that is similar to a dog barking. Phosphorus pentoxide is formed during the oxidation process (Ref.4):
P4 + 5O2 → 2P2O5
The chemical behavior of phosphorus is interesting because as a non-metal it exhibits ionic and covalent bonding depending on where it is present. It is an important element in complex organic compounds like ATP, (adenosine tri-phophate) as well as in simpler molecules like phosphoric acid ,H3PO4, oxides of phosphorus, or as a phosphate, PO43- in an inorganic compound. ATP is an energy-bearing molecule present in all living organisms. (Ref.5)(Fig.3)(Nuggets of Information)
The most common use of phosphorus is in the manufacture of fertilizers. It is also used in the production of steel and was used extensively in the manufacture of detergents, though lately phosphate-free detergents are more prevalent. This is because the phosphates that leached into water bodies affected it adversely.
There is much talk about peak phosphorus today. This means that our demand is exceeding the supply of phosphorus. In 2008, the world was shocked by an 800% increase in the price of phosphorus. Several factors including world reserves, agricultural practices using excessive amounts of manufactured phosphates have played a role in this shortage. As a society, we need to be cognizant of these problems and figure out ways to counteract the decrease in phosphorus. (Nuggets of Information)(Ref. 6)
Activities for Middle School Teachers:
Phosphorus has several allotropes. What other elements exhibit allotropism?
Study several biological processes that need ATP. (Nuggets of Information)
Study the reaction of phosphorus with the halogens( F, CL, I, Br, At) and balance the equations.
Nuggets of Information:
Phosphorus is named from the Greek phrase:’bringer of light’. (Ref.7)
Phosphorus exists as mainly three allotropes: red, white and black. The red and white form have a tetrahedral structure with 4 phosphorus atoms at each corner of the tetrahedron. (Fig.3) The black allotrope has a layer-like structure where one layer of P atoms are layered over another. The red allotrope has a hard crystalline structure and is non -poisonous and more stable than the white or yellow allotrope. The white P is soft and waxy, poisonous and spontaneously combusts in the presence of oxygen. Black P is the least reactive form and has a metallic lustre. (Ref.8)
Phosphoric acid is added to cola drinks to give it their sharp taste. (Fig. 7) Magnesium phosphide, Mg3P2, is used as warning flares and phosphorus, P, is used in Light Emitting Diodes or LEDs. (Fig. 7)
Knowing the combustible nature of white P, one of the most awful uses of White P has been in warfare. From World War 2, it has been used in tracer bullets, firebombs and in Middle East wars, where victims are burnt in horrific ways. Lately P has been used to make nerve gases like Sarin and was released in a Tokyo subway in 1995. White P was also used to make matches in the 19th century, but no-one realized how deadly the vapours were till the young girls working in the factories developed phossy jaw, which ate the jaw bone. (Fig. 7)
As mentioned earlier, too much P in water can make it a pollutant, choking the biological life in streams and lakes. What happens is that there is too much plankton growing that kills fish and other water life. There is also prevention of sunlight from reaching lower levels of the water, thereby killing several species there as well. This process is called eutrophication. (Ref. 9)
Just like there is a nitrogen cycle in nature, we have a phosphorus cycle as well. It differs from other cycles because the gaseous phase is minimal. Most of the phosphates are found in sedimentary rocks. When it rains, the process of erosion and weathering distributes the P in the soil and water. Plants absorb the phosphates and animals eat the plants. The phosphates are returned to the earth and rocks through the urine and feces from animals, as well as during decomposition and death of animal and plant matter. Aquatic eco systems also imbibe the phosphates through sewage seepage, fertilizer runoff and from industrial wastes. Animals eat the plants and the process is continued as before.
Phosphorus is found in a lot of foods we eat. Many lentils, meats and fish like salmon contain P. In addition, a lot of nuts like Brazils, cashews and almonds contain P.(Ref.10)
Adenosine-Tri-Phosphate, or ATP is the energy currency in several cell mechanisms. During respiration and exercising, which means almost all the time, ATP is necessary for all living beings. The structure of ATP has an ordered carbon compound as the main part; however the phosphorus is critical. Three of the phosphorus atoms are linked via oxygen atoms and there are extra oxygen atoms attached to each of them as well. The negative charges on the oxygen when the hydrogen leaves as a proton on the hydroxyl groups, OH, makes them have high potential energy. This means they are not stable and are willing to lose a phosphate ion from an end to form ADP, Adenosine-Di-Phosphate. The conversion of ATP to ADP releases about 7.3 Kcal per mole. Living things use ATP like a battery, since it powers reactions by losing a phosphate and becomes ADP. Food energy converts the ADP once again to ATP and the process is repeated. (Fig.3)(Ref. 11)



References:
- https://www.nlm.nih.gov/medlineplus/ency/article/002424.htm
- http://www.scienceshorts.com/articles/Discovery%20of%20Phosphorushtm
- http://www.chemicool.com/elements/phosphorus.html
- http://dwb5.unl.edu/CHEM/Redox/RedoxA13.html
- http://science.howstuffworks.com/dictionary/plant-terms/adenosine-triphosphate-atp-info.htm
- http://www.sciencedaily.com/releases/2014/02/140203083830.htm
- http://www.rsc.org/periodic-table/element/15/phosphorus
- http://chem-guide.blogspot.com/2010/04/allotropes-of-phosphorous.html
- http://enviroliteracy.org/air-climate-weather/biogeochemical-cycles/phosphorus-cycle/
- http://lifestyle.iloveindia.com/lounge/phosphorus-rich-foods-3844.html
- http://hyperphysics.phy-astr.gsu.edu/hbase/biology/atp.html